1、 Case company introduction

Shiyao Yinhu Pharmaceutical Co., Ltd. is a subsidiary of Shiyao Group, mainly engaged in the production of formulated products. It is the main production, sales, and research and development base for the group's large and small volume injections and oral preparations. The company covers an area of over 600 acres, with a registered capital of 150 million yuan and more than 900 employees. It is a Shanxi Provincial Enterprise Technology Center, Provincial High tech Enterprise, Provincial Excellent National Economic Mobilization Center, and Municipal Key Laboratory.
2、 Project Background
Shiyao Yinhu Pharmaceutical Co., Ltd. has deployed an online cleanliness monitoring system in the production process of small volume injections. Monitoring points for particles and planktonic bacteria have been installed in key processes, background areas, and other areas of the glass ampoule filling lines from line one to line five. A total of 23 particle and 18 planktonic bacteria monitoring points have been installed to ensure that the production environment meets GMP Class A (Grade 100) and Class B (Grade 1000) cleanliness standards, meets GMP related requirements, and guarantees product quality.
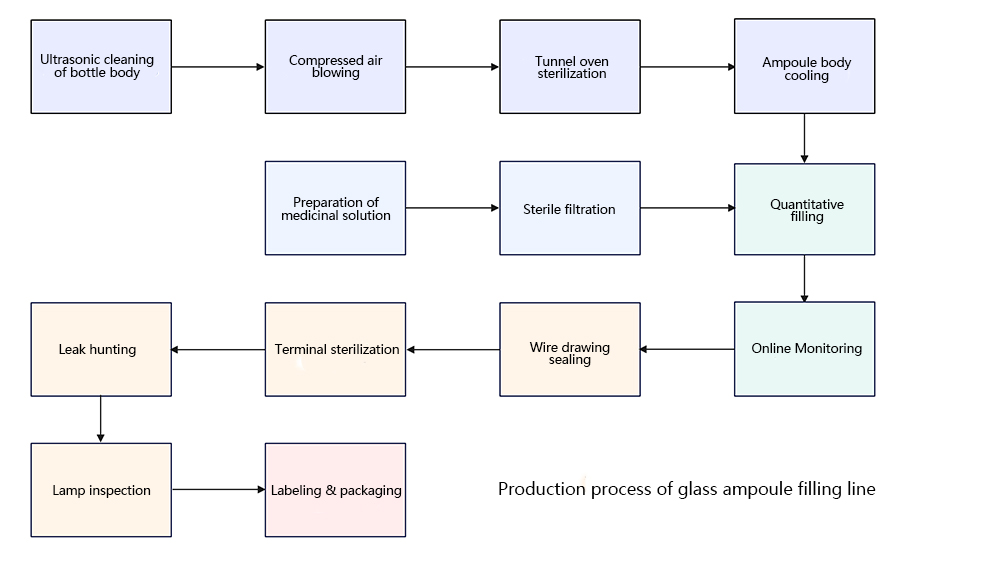
3、 Core Challenge
|
Key control points of production process |
||
|
Production processes |
Key control parameters |
GMP requirements |
|
Ampoule cleaning |
Quality of injection water and ultrasonic power |
Particles ≤ 25 per bottle |
|
Tunnel oven |
Temperature ≥ 300 ℃, time ≥ 5 minutes |
Endotoxins decrease by ≥ 3 logs |
|
Bottling |
Loading accuracy ± 1%, A-level wind speed 0.45 ± 0.1m/s |
Sterility assurance level (SAL ≤ 10 ⁻⁶) |
|
Sealing-off |
Flame temperature, sealing integrity |
Leakage rate ≤ 0.1% |
|
Sterilization |
F₀ value ≥ 15 (wet heat sterilization)) |
Biological Indicator Challenge Test |
Continuous online sampling of key areas
During the entire production process, real-time continuous sampling is required, with certain requirements for the installation location of points and the performance of instruments. Key production areas (A/B level clean areas) implement 24-hour continuous sampling.
Limitations of manual sampling
Traditional handheld particle counters require a long time for single point sampling and cannot cover the entire production process. At the same time, manual sampling requires frequent entry into clean areas, which increases the risk of personnel intervention and may affect environmental stability. Moreover, the timeliness is poor, the detection results lag behind, and it cannot reflect environmental changes in real time, making it difficult to support rapid decision-making.
Difficulty in tracing
Without electronic maps, sudden pollution incidents cannot be located in real time, and manual processing can easily lead to tampering or missing historical data, resulting in delayed root cause analysis.
4、 Solution: Intelligent online monitoring system
1. System architecture
Perception layer:
Deploy laser particle counters (0.5 μ m/1.0 μ m/5 μ m/10 μ m) and planktonic bacterial samplers;
Transport layer:
Adopting shielded twisted pair cables to strictly follow the hand in hand topology, avoiding star/fork connections to sensors, and achieving data transmission through Modbus/RS485 protocol;
Platform layer:
EMS online monitoring platform integrates SPC statistical analysis and over limit warning (sound and light alarm);
Data analysis chart
Interaction layer:
On site billboard display+multi-level permission management.

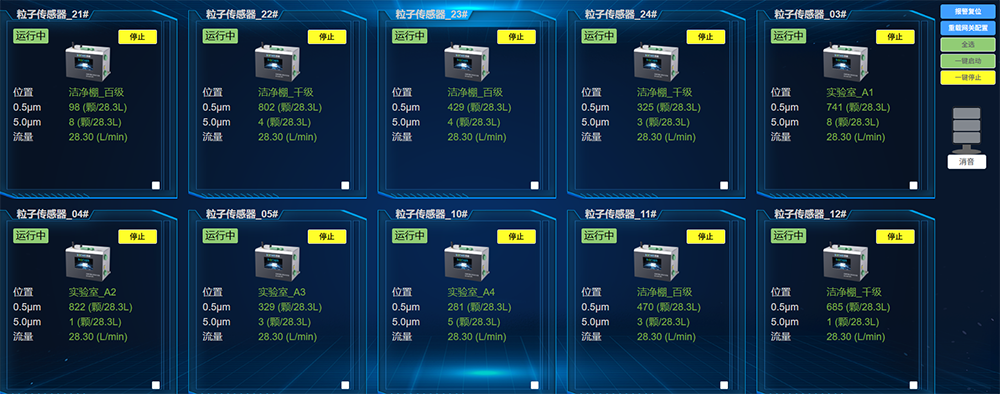
2. Key technologies
Excellent optical system: Using self-developed customized composite lenses to manufacture highly averaged strip-shaped light spots, the energy size of particles entering the photosensitive area is consistent, and counting is more accurate;
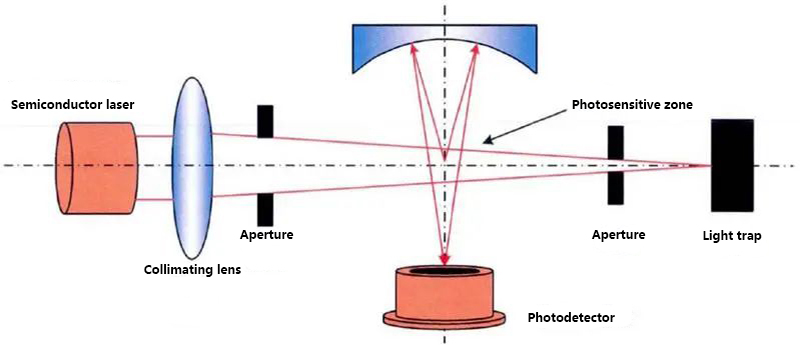
Principle diagram
Average stripe spot
A stable and pollution resistant flow detection method: utilizing the characteristics of the flow channel to design a dedicated structure to achieve accurate flow measurement, solving the problem of easy pollution and inaccurate measurement of thermal flow sensors after a period of operation, and improving the stability and consistency of flow measurement;
Multiple anti-interference technologies: optimize the ultra-low noise power supply system to control power supply noise within ± 5mV; By designing specialized shielding components, external interference shielding of sensor core components can be achieved; Referring to electromagnetic compatibility design specifications, effectively introducing static electricity and electromagnetic compatibility noise from the shell into the ground;
Add interface filters to effectively suppress electromagnetic interference and noise and optimize signal transmission quality;
Digital twin: Linking environmental data with production batch numbers to achieve precise correlation between product batches and pollution events.
5、 Implementation effect
1. Significantly reduce labor costs without requiring team members to fix daily measurement data;
2. Real time monitoring and automatic uploading of data, eliminating the need for manual data entry and eliminating doubts about data fraud;
3. Provide real-time monitoring data of the entire lifecycle environment based on different batches of products to enhance the credibility of the enterprise;
4. Continuous monitoring avoids the problem of data lag in manual sampling and facilitates timely handling of anomalies;
5. Based on environmental monitoring data, effectively formulate environmental improvement measures;
6、 Industry innovation points
Multi parameter fusion monitoring
Incorporate particle concentration, temperature and humidity, and wind speed into the same evaluation model to construct a pharmaceutical specific cleanliness index (LCI).
Multi parameter analysis model
Simultaneously analyzing parameters such as particle concentration, temperature and humidity, and wind speed provides effective data support, which has a significant effect on clean room management and environmental improvement.
Compliance management
Automatically generate audit trail reports that comply with FDA 21 CFR Part 11 standards to meet overseas customer factory inspection requirements.
Data integrity
Support breakpoint continuation, after communication is restored, data is uploaded to the platform in an orderly manner, ensuring data integrity.
7、 Conclusion
This case validates the core value of the online cleanliness monitoring system in the production process of small volume injections: through real-time data and continuous monitoring functions, it provides effective and powerful data support for the environmental status of the entire production process, enhancing the credibility of the enterprise. Effective assistance has been provided in formulating environmental management measures, which helps to improve the qualification rate and quality of products.

Central Control Room

Filling workshop interactive screen

Filling Workshop - Key Area 1

Filling Workshop - Key Area 2

Filling Workshop

B-level background area

B-level background area

Filling line

HMI

Airborne bacteria

Bottle out

Bottle out

Bottling



